I Tested a Mitochondrial Peptide That Was Supposed to Raise Testosterone
A first-in-human test that narrowed the hypothesis
What a rigorous N-of-1 trial actually showed
Short version: Two investigational peptides targeting mitochondrial steroidogenesis were safe but did not meaningfully raise total testosterone at the tested dose. One showed a tentative signal on free testosterone. The null result is likely real, and informative.
Context
If you want the full background on why mitochondrial cholesterol transport matters, how VDAC1 and 14-3-3ε regulate steroidogenesis, and why these peptides looked promising preclinically, I covered all of that in detail here:
This post is about what actually happened when I tested the idea in a controlled human experiment.
The Question This Study Actually Asked
Stripped of theory, the experiment boiled down to this:
If you remove an inhibitory constraint on Leydig-cell steroidogenesis, does testosterone meaningfully increase in a real human under tightly controlled conditions?
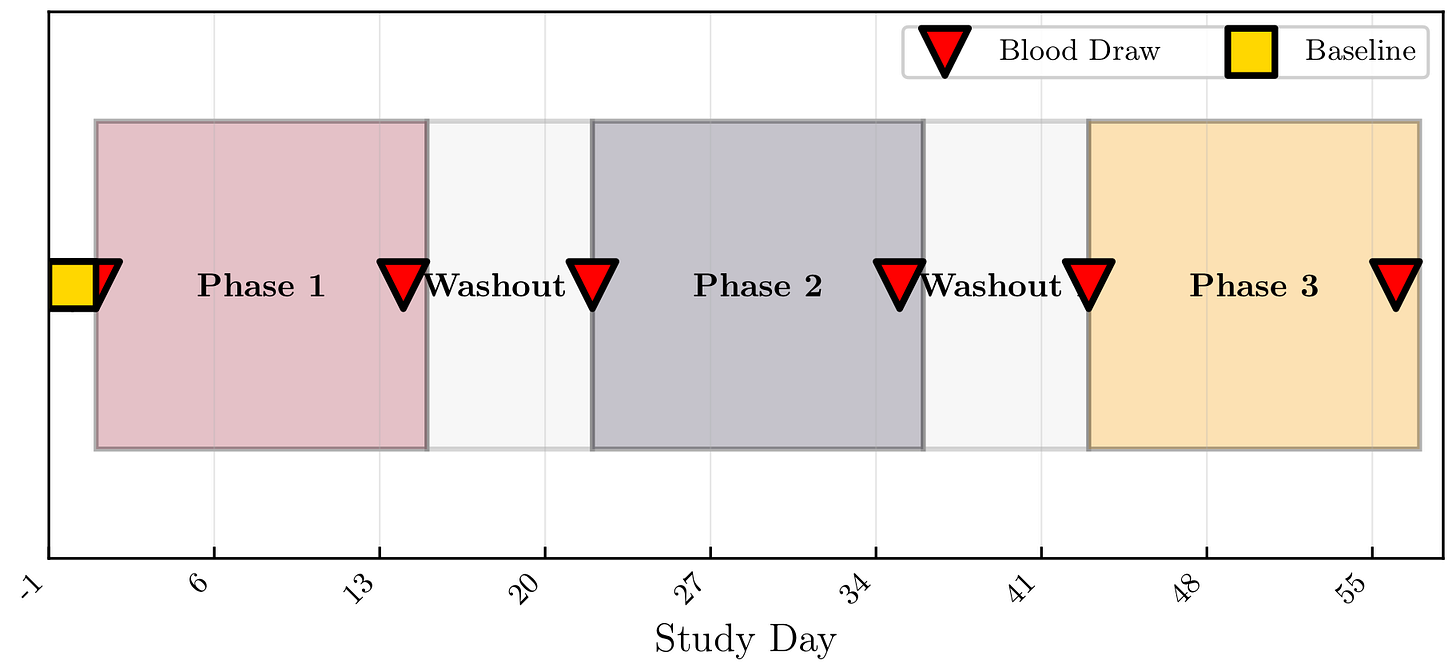
Study Design
Design: Randomized, double-blind, placebo-controlled N-of-1 crossover
Interventions:
ACE-167
N163-166 Mod. 6
Placebo
Dose: 30 mcg/day (deliberately conservative, first-in-human)
Phases:
3 × 14-day treatment periods
7-day washouts
Primary endpoint: Morning fasting total testosterone (LC-MS/MS)
Secondary endpoints:
Free testosterone (equilibrium dialysis)
LH, SHBG, estradiol
Daily patient-reported outcomes
Analysis: Bayesian pooled-replicates model (appropriate for N-of-1 inference)
If you want the full protocol, priors, diagnostics, and sensitivity analyses, those live in the manuscript.
🧪 Study Timeline
The Participant
Healthy 27-year-old male
Baseline testosterone: 943 ng/dL (>90th percentile)
Stable sleep, diet, activity
No alcohol, no endocrine disease
On finasteride (stable for >2 years)
This was admittedly not an optimized population for seeing large gains.
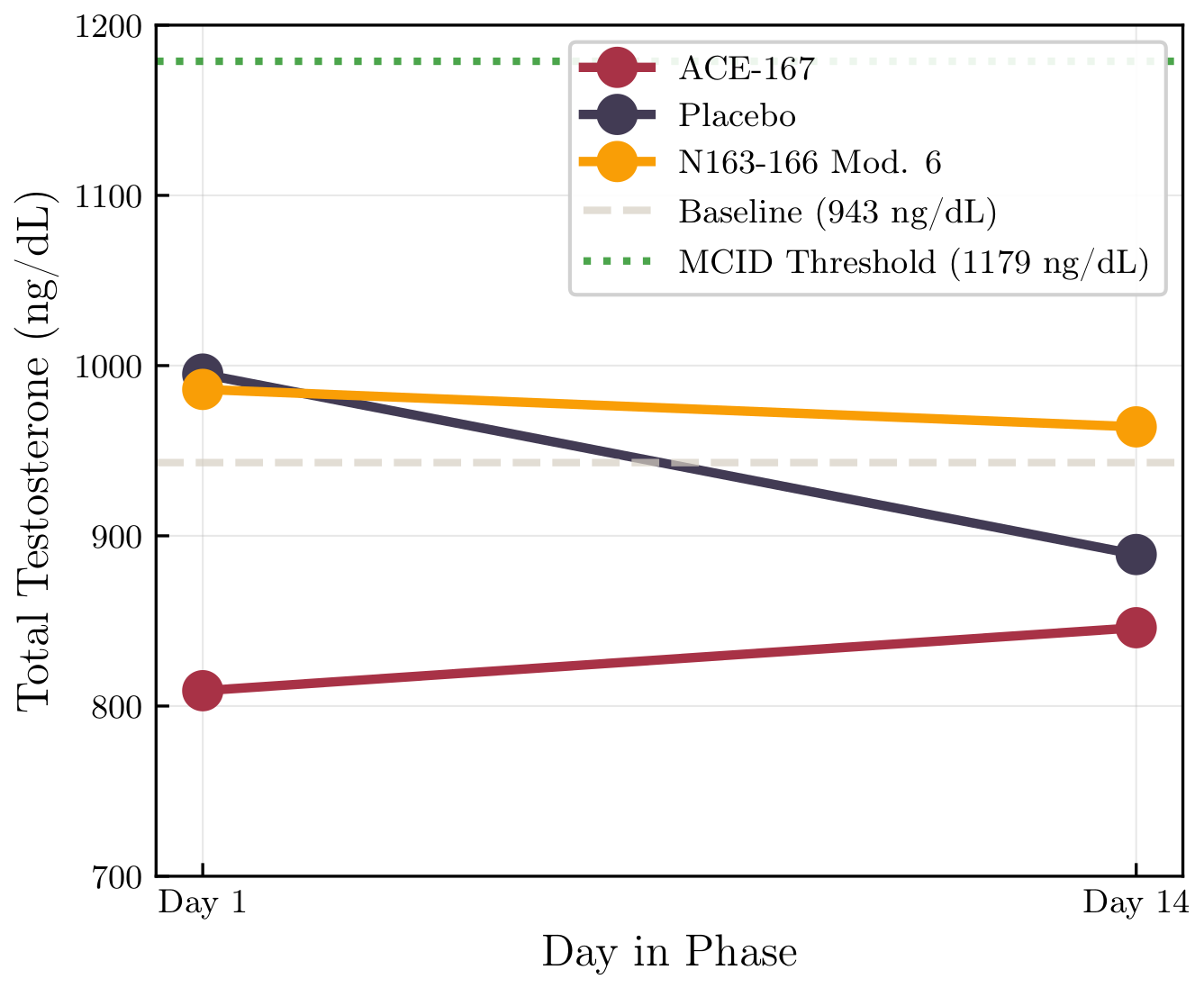
Primary Result: Total Testosterone
Neither peptide meaningfully increased total testosterone relative to placebo.
Bayesian treatment effects vs placebo:
ACE-167: −102 ng/dL
P(Δ > 0) = 0.06
N163-166 Mod. 6: +34 ng/dL
P(Δ > 0) = 0.73
The probability of exceeding a clinically meaningful increase (~+25%) was essentially zero for both.
Timing caveat: blood draws were fixed at ~2 hours post-dose, extrapolated from rodent oral-dosing data. If peak effects in humans occur earlier, later, or require longer accumulation, this design would miss them. The null result therefore applies specifically to effects present at the 2-hour window.
🧪 Testosterone Trajectories
Why This Null Result Is Probably Real
Two explanations dominate, and they’re not mutually exclusive.
1. Dose was likely too low
The human dose was ~15× below the maximal effective rodent dose after scaling. This study prioritized safety and falsifiability and didn’t attempt to find the maximal effect.
2. Baseline testosterone was already near ceiling
These peptides remove inhibitory processes; they don’t drive production. If Leydig cells are already operating near physiological limits, there may simply be little headroom.
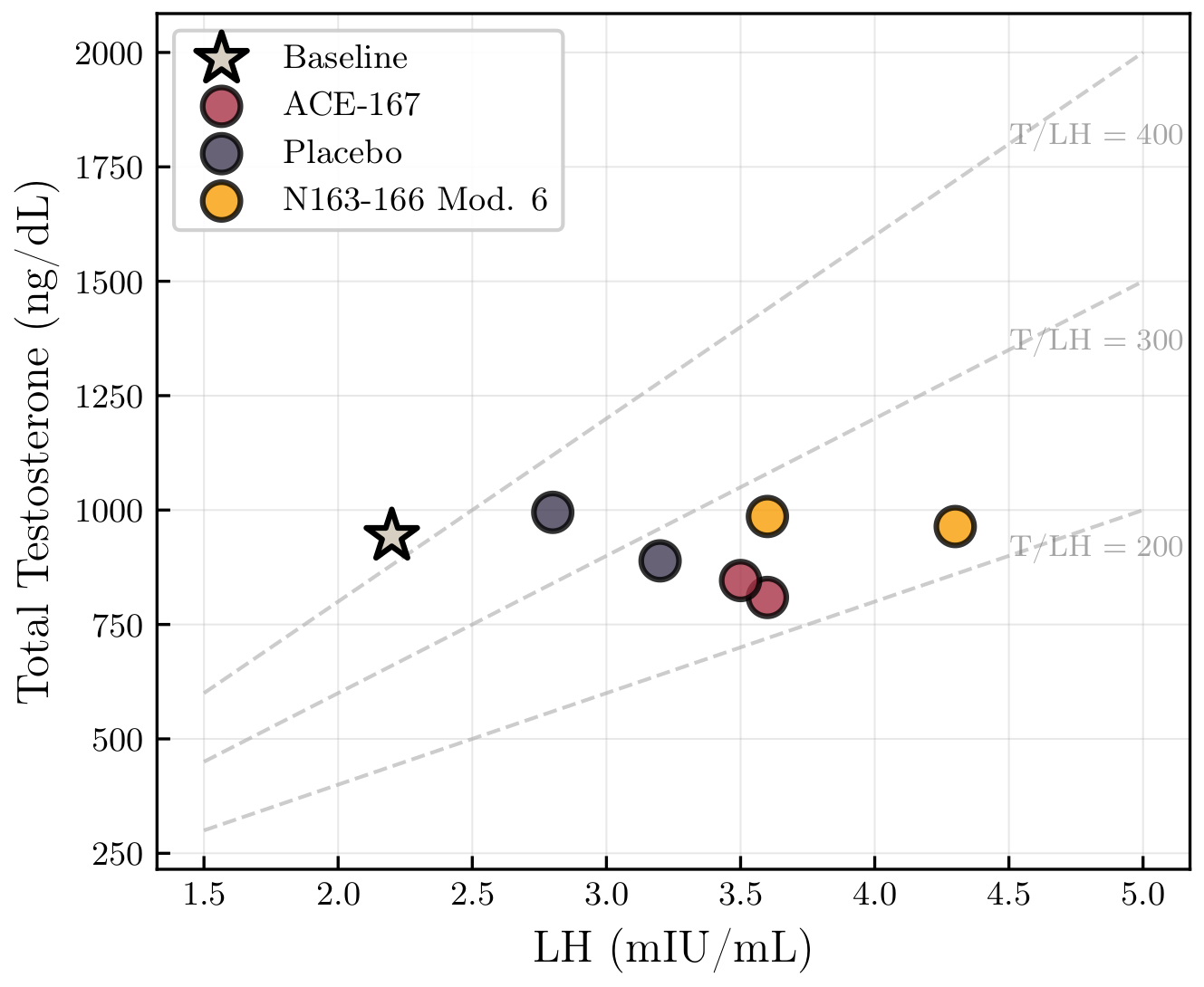
A Mechanistic Clue: LH Went Up, Testosterone Didn’t
Across all phases, including placebo:
LH increased substantially
Testosterone stayed flat
The testosterone-to-LH ratio fell by ~25–45%
🧪 Testosterone vs LH
This pattern is consistent with limited Leydig cell responsiveness at high baseline androgen levels. More signal, same output.
Because LH is highly pulsatile, sparse sampling limits interpretability; observed differences may reflect timing rather than treatment.
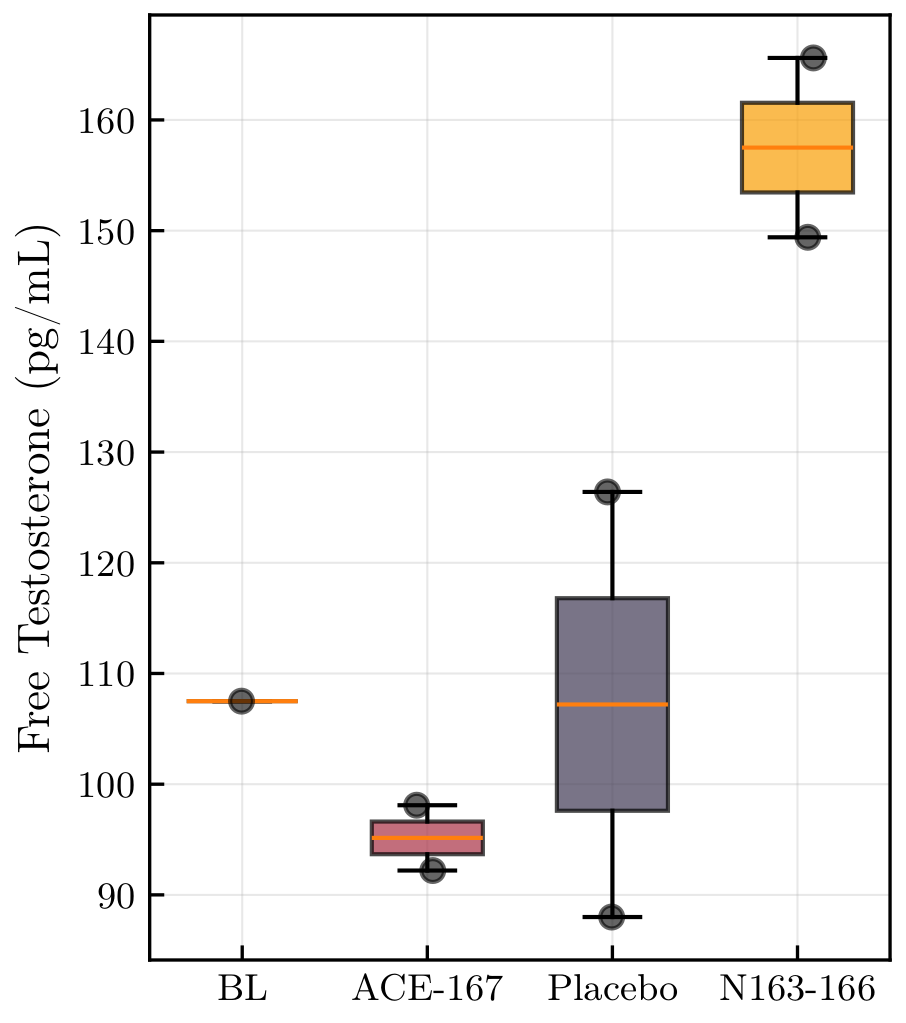
The One Signal Worth Discussing: Free Testosterone
Total testosterone was null.
But under N163-166 Mod. 6, free testosterone showed a directional increase:
+47% vs baseline
Bayesian estimate vs placebo:
+37.5 pg/mL
P(Δ > 0) = 0.97
🧪 Free Testosterone by Phase
Important Caveat
Only two measurements per phase
Equilibrium dialysis has meaningful analytical and biological variability
Expected reference change value ≈ 40%
This could be a real signal, or pure noise. Mechanistically, a selective free-testosterone increase without total-T change is hard to explain for this compound class.
Replication with denser sampling is required.
Subjective Outcomes: Interesting, Limited Interpretability
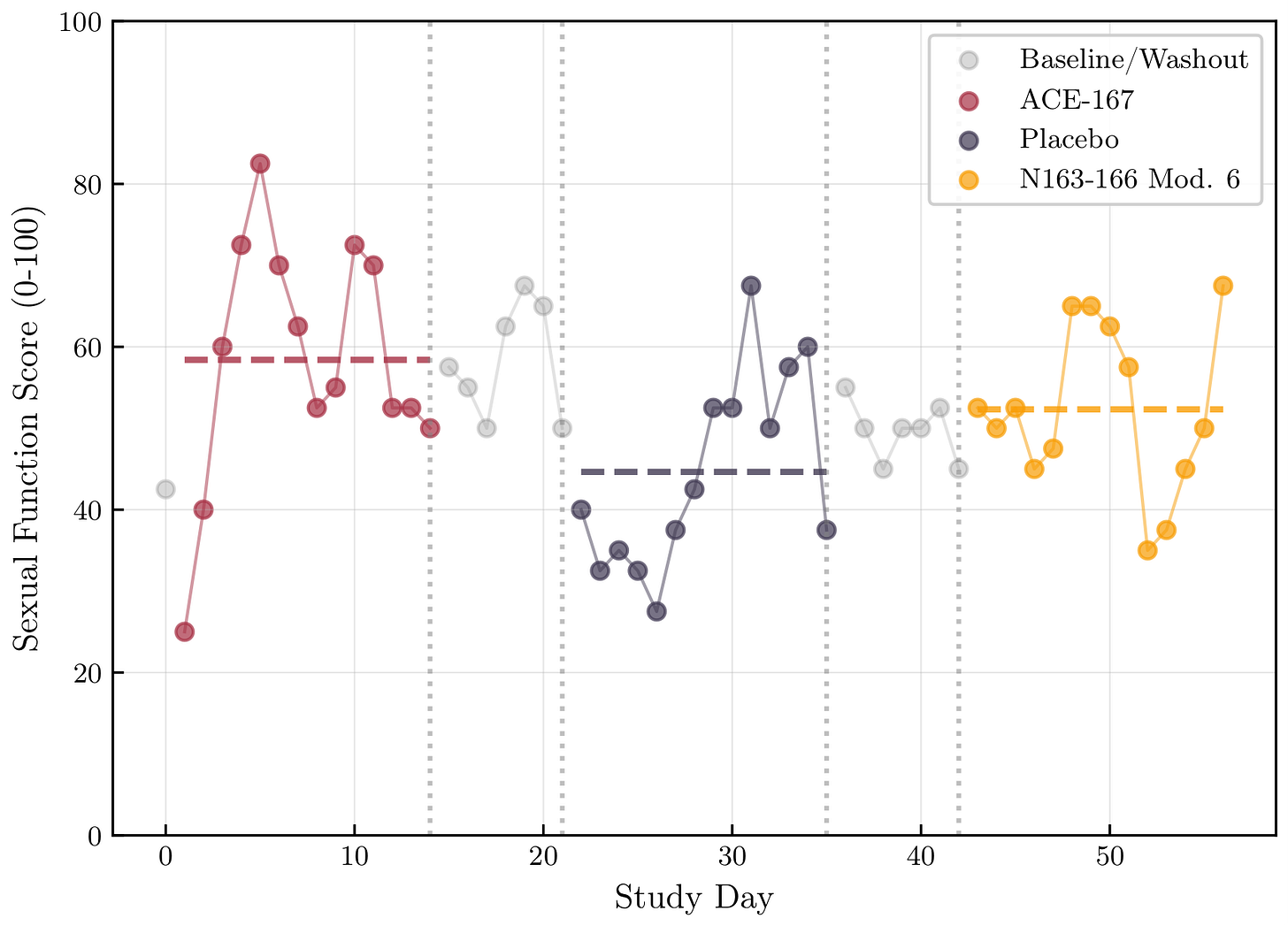
ACE-167 showed higher scores for sexual function and sleep.
But:
ACE-167 was Phase 1
Strong phase-order and expectancy confounding
Autocorrelated daily measures
Multiple comparisons give randomness a leg up
These are hypothesis-generating only.
🧪 Sexual Function Timeline
Safety
No adverse events
No hematocrit rise
No BP, liver, or metabolic issues
At 30 mcg/day, both peptides were well tolerated, paving the way for future work
What Comes Next (If Anything)
A serious follow-up would need to:
Use higher doses (60–150 mcg/day)
Enroll lower-baseline participants (500–700 ng/dL)
Exclude finasteride
Sample hormones much more frequently
Use adaptive Bayesian stopping rules
Whether that’s worth doing is now a much better-informed question than it was before this study.
Bottom Line
This experiment didn’t validate the peptide hypothesis, but it didn’t kill it either.
And in physiology, bounding a hypothesis is often more valuable than chasing another uncontrolled “positive” result.
See below for the full experimental outline and results. Peptide synthesis and supply was provided by Upgraded Bio (admin@upgraded.bio).